

Biodegradable and non-biodegradable polymers are two categories constituted of large molecules. These polymeric substances are those macromolecules formed by the linkage of smaller repeating units called monomers. Polymers like cellulose, proteins, nucleic acids, paper, glass, plastic, and rubber are some of its examples. These large molecules and polymers are broken down into simpler molecules under two conditions - naturally or synthetically. Natural change is the biological activity of enzymes, and synthetically undergoing changes are due to adding chemicals to these polymers. The process of degradation or breaking down of large molecules into simpler ones under such conditions is called decomposition. Polymers experience these changes entirely depending on their bonding and structure.
These polymers are materials that undergo physical and chemical changes under aerobic and anaerobic conditions when exposed to microorganisms/enzymes. It forms by-products such as carbon dioxide, water, inorganic salts, and biomass. Biodegradable polymers undergo degradation through two processes:
Enzymatic process - In this process, degradation occurs through the enzyme actions of microorganisms like bacteria, algae, and fungi.
Non-Enzymatic process - In this process, degradation occurs through chemical hydrolysis that decomposes the chain of these polymers.
Therefore, biodegradable polymers are substances that undergo decomposition naturally.
A few examples include-
Poly-β-hydroxybutyrate-co-β-hydroxy valerate (PHBV) - It is a copolymer of 3-hydroxy butanoic acid and 3-hydroxy pentanoic acid, in which an ester group cross-links monomers.
Polyglycolic acid (PGA) - It is obtained by chain polymerization of a cyclic dimer of glycolic acid, OH−CH2COOH
Polylactic acid (PLA) - Polymerization of the cyclic dimer of lactic acid (OH−CH(CH3)COOH) .
Poly (ε -caprolactone) (PCL) - It is obtained by chain polymerization of the lactone of 6-hydroxy hexanoic acid
Nylon-2-Nylon-6 - It is a polyamide copolymerization of glycine (NH2CH2COOH) and aminocaproic acid (H2N−(CH2)5−COOH) .
These polymers are not degraded or digested by the action of microorganisms. It consists of long chains of carbon and hydrogen atoms with stronger interionic bonds between them. They are causing harm to the environment as their ability to remain intact over the years. Also, these polymers entered our food chains.
Polypropylene(PP)
Synthetic rubber
Polyvinyl chloride(PVC)
Polyethylene(PE)
Polythene terephthalate(PET)
Polystyrene(PS)
| S.No | Biodegradation Polymers | Non-Biodegradation Polymers |
|---|---|---|
| 1 | It decomposes naturally by the action of microorganisms. | It doesn't decompose naturally. |
| 2 | It consists of short chains. It consists of amide, ester, and ether bonds. | It consists of long chains of carbon and hydrogen atoms. |
| 3 | These chains are hydrolytically or enzymatically cleaved. | These chains are chemically inert and hence not able to decompose. |
| 4 | These polymer molecules have weak linkages of bonding. | These polymers have an interionic type of bonding. |
| 5 | A short period of decomposition is required. | A long duration of decomposition is required. |
| 6 | It consists of raw materials and renewable resources. These polymers are environmentally friendly. | It comprises dangerous plastics, aluminium cans and bottles, scrap metal, foam styrol, tires, paints, and various chemicals. These polymers are harmful to the environment. |
These polymers are present naturally and synthetically, consisting of functional groups like amide, ester, and ether. It undergoes many reactions for its formationcondensation reaction, ring-opening polymerization, and a metal catalyst.
The hydrolyzable ester groups react to the polymer chain resulting in a weak ester link and making it attacked by enzymes. Such polymers are biodegradable polymers. Also, the properties and breakdown mechanisms are determined by their exact structure.

The mechanism for the formation of biodegradable polymer
There are two types of polymers −
Biopolymers and renewable resources − These polymers comprise renewable resources and natural raw materials. These are produced directly by biomass, microorganisms, and bioderived monomers.
| Biomass examples | Microorganism examples | Bio-derived monomers examples |
|---|---|---|
|
|
|
Petrochemical resources or non-renewable resources − These are produced from non-renewable resources or petrochemical products. A few examples include Poly (caprolactone), Poly(L-lactic acid), Polyglycolic acid, polyorthoesters, and Polyanhydride.
These polymers consist of long chains of carbons and hydrogen atoms. These polymers have an interatomic type of bonding and are strong enough for microorganisms to break the bonds and digest them and require a long period to decompose them. One commonly used non-biodegradable substance is polythene or plastic due to its low cost, versatility, and toughness. It is unaffected by the action of microorganisms. Therefore, some chemical reagents are required to decompose these polymers.
Polythene or Poly(ethene) results from the polymerization of monomers like ethylene or ethene. It undergoes a radical mechanism under high temperature and pressure with organic peroxide radical as an initiator. It is known as an additional polymerization reaction.
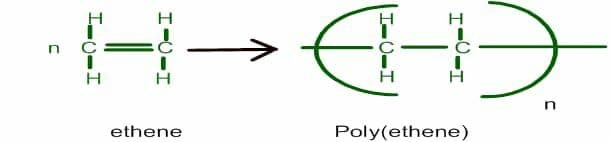
One large class of non-biodegradable polymers is Polyethylene or Polythene. This polymer has various types contributing to hardness, flexibility, stiffness, and chemical and moisture resistance. Different types include.
Linear high-density polyethylene (HDPE)
Linear low-density polyethylene (LLDPE)
Ultra-high molecular weight polyethylene (UHMWPE)
Chlorinated Polyethylene (CPE)
Medium-Density Polyethylene (MDPE)
Cross-linked Polyethylene (PEX)
Branch of low-density polyethylene (LDPE)
It is required to degrade every material, as it causes waste which is needed to decompose either naturally or synthetically. Processes like degradation majorly help reduce the waste by action of bacteria/enzymes. Some substances broken down into simpler substances by the action of micro-organisms are called biodegradation and are unable to break down naturally, called non-biodegradable. Most importantly, aluminium cans, plastic bottles, scrap metal, paints, tires, paints, and various chemicals, are needed to decompose as these substances are unaffected by natural processes and cannot be disassembled or degraded after thousands of years. They are highly toxic to the environment and contribute significantly to solid waste, which is harmful to human health.
Q1. What are the three advantages of biodegradable polymers?
Ans. Some advantages include-
It reduces carbon emissions.
It consumes less energy during its manufacture.
It is easy to recycle.
Q2. Given one disadvantage of biodegradable polymers?
Ans. It is cost-effective, as landfills in such a way that it is to be moisture-free and airtight to store potentially dangerous materials, which helps to prevent toxic chemicals from being released from landfills and often slows down biodegradation.
Q3. What are the properties of biodegradable polymers?
Ans. These polymers degrade in the presence of sunlight and radiation. Therefore, it has low melting and degradation temperatures. Elasticity and durability are generally low in these polymers. Also, it has low strength and hardness compared with metals and ceramics.
Q4. How do non-biodegradable substances affect the environment?
Ans. These substances accumulate in the environment, do not decompose naturally, and remain in the ecosystem, which in return, causes water and soil pollution.
Qns. 5. What long-term effects does the use of non-biodegradable products have in the end?
Ans. Continuous exposure to synthetic materials in the presence of sunlight, water, and air causes the release of highly toxic pollutants that can enter the water, thus causing water pollution