

The organic compounds that form a series that consists of the same functional groups but have variations in their $\mathrm{-CH_2}$ group are determined as the homologous series. In this homologous series, various successive compounds can be seen including alcohol, phenol, ether, and so on. The alcohols have more than one functional group that is quite similar to the functional groups of phenols and ether.
The organic compound in which the atom of hydrogen or the aliphatic carbon gets replaced by the hydroxyl group is referred to as the alcohols. The compound can be identified by its sweet smell. Alcohols can be of 4 types: ethyl, denatured, isopropyl and rubbing alcohols. In all these, alcohol types, ethyl alcohol are widely used which can also be referred to as grain alcohol or ethanol. Ethyl alcohol is widely used for the preparation of beverages like wine, beer, and other liquors. Alcoholic beverages are generally made with yeast and fermented sugar.
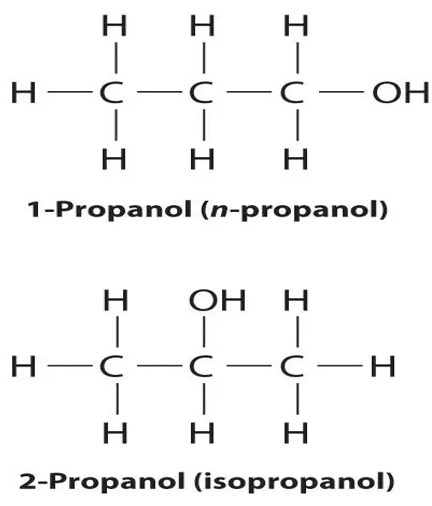
The major simple forms of alcohol include methanol $\mathrm{(CH_3OH)}$, ethanol $\mathrm{(C_2H_5OH)}$, propanol $\mathrm{(C_3H_7OH)}$ and butanol $\mathrm{(C_4H_9OH)}$.
The structure of alcohol is generally attributed due to the presence of the hydroxyl group. The atom of carbon that is present in the molecule of alcohol the atom of carbon remains bonded to the oxygen atom of the hydroxyl group with the help of a sigma bond. The sigma bond is usually denoted by the symbol σ.
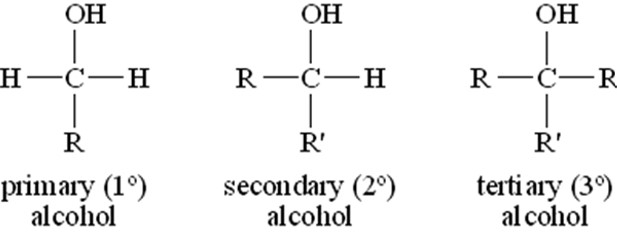
Figure 1: Structure of Alcohols
The bond in the alcohol molecule is created because the overlap of an sp3 hybridized orbital of carbon takes place with an $\mathrm{sp^3}$ hybridized orbital of oxygen. Repulsion takes place between the oxygen electron pairs that remain in an unshared state.
Various types of alcohols can be used for several medical, industrial, and technical purposes as discussed below.
Ethanol is also referred to as the intoxicating component that is utilised in the preparation of various alcoholic beverages including, distilled spirits, wine, and beer. The molecule formula of ethanol can be written as $\mathrm{C_2H_5OH}$. It has a density of 789 kg/m3 and a molar mass of 46.07 gm/mol. The properties of ethanol highlight that, it is a courless liquid having a burning taste and sweet smell.
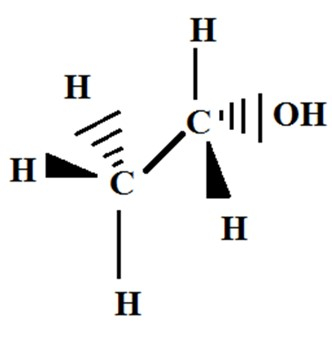
Figure 2: Structure of Ethanol
Propanol can also be referred to as Isopropyl alcohol that is prepared through indirect hydration of propylene. Propanol or Isopropyl alcohol can be used as rubbing alcohol and solvent in various cosmetic and medical industries that be applied to the skin. Propanol is more toxic than ethanol or ethyl alcohol.
Glycerol also determined as glycerine, is the sweet syrup that has 3 alcohol hydroxyl groups. Propane-1,2,3-triol is the systematic name of glycerol which is generated through sugar and molasses fermentation. A large amount of glycerol is required in order to prepare glyceryl trinitrate (nitroglycerin).
The common chemical and physical properties of alcohols are stated below.
Reaction with phosphorus halide − Alcohols get reacts with Phosphorus halide and prepare haloalkanes. The reaction can be written as: $\mathrm{ROH + PCl_5\:\rightarrow\:R-Cl + POCl_3 + HCl}$.
Reaction with Grignard reagent − Alcohols get react with Grignard reagents in order to produce hydrocarbons. The reaction can be written as: $\mathrm{CH_3OH + C_2H_5MgBr \:\rightarrow\:C_2H_6 + CH_3OMgBr}$.
Reaction with thionyl chloride − In pyridine, alcohols are treated with thionyl chloride to produce chloroalkanes. The reaction can be stated as: $\mathrm{ROH + SOCl_2\:\rightarrow\:R-Cl + SO_2\uparrow + HCl\uparrow}$ .
Higher alcohols get solidified at room temperature whereas lower alcohols remain in the liquid state.
Alcohols have a higher boiling point than haloalkanes that remain associated with intermolecular hydrogen bonding.
The boiling point of the alcohol compound decreases due to decrease in the surface area.
Solubility of alcohols in water reduces with the increase in their molecular mass and due to a reduction in the extent of intermolecular hydrogen bonding.
Some common applications or uses of alcohol are stated below.
Alcohol like Ethanol and methanol can be used as fuel to operate automobiles.
Consumption of alcoholic beverages can help in lowering the blood glucose level and act as a temporary pain reliever for humans.
Ethanol is also referred to as a type of alcohol widely used in several medical sectors as an intoxicating or anaesthetic agent.
One of the vital industrial chemicals is ethanol that is generally used as a solvent in order to synthesize other organic chemicals. It is also used as an additive to gasoline which is the component of refined petroleum and is used in automobiles and jets.
The compound that is derived when 1 atom of hydrogen gets replaced by a hydroxyl group within an aliphatic hydrocarbon is referred to as alcohols. Alcohol can be used for various industrial and medicinal purposes. In food and beverage industries the alcohols used in the preparation of beverages like wine, beer, whiskey, and other liquors occur widely after adding a little amount of yeast and fermented sugar in it.
Q1. What is ethanol?
Ans. Ethanol is a chemical compound, that can be also determined as the ethyl alcohol, alcohol, or grain alcohol, a major member of the alcohol group. The molecular formula of ethanol can be written in the form of $\mathrm{C_2H_5OH}$.
Q2. What the structure of alcohol compounds denotes?
Ans. The molecule of alcohol generally consists of two major parts including the alkyl group and hydroxyl group. The bond angle that can be seen in the case of an alcohol molecule is the $\mathrm{C-O-H}$ bond angle, which remains a slightly tetrahedral angle (109°-28′).
Q3. What are properties of alcohols?
Ans. Alcohols generally react with hydrogen halide in order to prepare alkane halide. The reaction can be written as $\mathrm{ROH + HX\:\rightarrow\:RX + H_2O}$ .The physical properties of alcohols denote that alcohol's acidic strength gets reduced with the electron number and with the increase of the donating carbon groups.