

Alkanes are considered important as these are crucial raw materials predominantly necessary in the chemical industry. More to this, alkanes are used as a primary ingredient in the gasoline as well as in lubricating oils. One of the most crucial properties of alkanes is combustion that states a condition when in the presence of oxygen, alkane’s burn forming the resultant products carbon dioxide as well as water.
Alkanes are known as paraffin and these are organic compounds that consist of both the atoms of carbon and hydrogen. Covalent bond is noted among alkanes that display a structure that is tree-like.
Figure 1: Structure of Alkanes
Fvasconcellos 20:06, 8 January 2008 (UTC). Original image by DrBob contribs)., IUPAC-alkane-5, CC BY-SA 3.0
The formula that is followed by alkanes is expressed as, CnH2n+2 respectively, and possesses both physical and chemical properties specific to the numbers of bonds present with a number of atoms of carbon and hydrogen. The most popular structure of alkane is methane that is CH4. Alkanes are saturated hydrocarbons having sigma bonds.
Physical properties are the characteristic features of a substance are easily measured as well as observed, without any changes in the identity of the substances. For alkanes, physical properties involve its respective structure, nature of solubility, and boiling and melting points stated as follows −
In the compound of alkanes, the atoms of carbon present display the form of sp3 hybridised. This has four sigma bonds that occur between the carbon and hydrogen atoms.
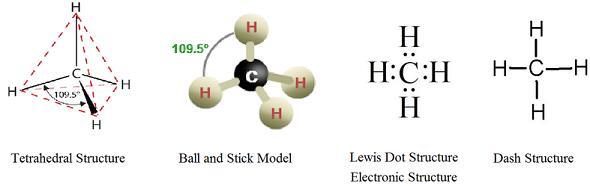
Figure 2: Electronic Structure of Alkanes
1840460mahesh, Alkanes structers 3, CC BY-SA 4.0
The structure of methane is tetrahedral and symmetric in nature. The bond angles are 109.47°.
The solubility of alkanes depends on the bonding of the atoms of hydrogen and carbon. There lays a little difference thereby resulting in the occurrences of electronegativity and they have a bond that is covalent in nature. The alkanes are non-polar in nature and suggest solubility in non-polar solvents. Alkanes are said to be hydrophobic and are not soluble in water.
The boiling point of alkanes is stated by the Van Der Waals intermolecular forces. This force tends to increases with the increase in the size of molecules, or with the “increase” in the surface area.
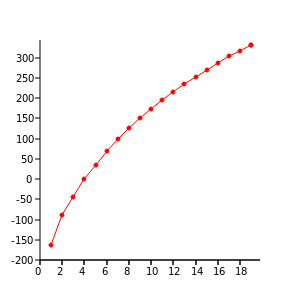
Figure 3: Boiling Point
AgnesLager, Alkane boilingpoints (centigrade) from methane to nonadecane, CC BY-SA 4.0
The boiling point of alkanes is stated to increase depending on the increased weight of the weight of the molecule
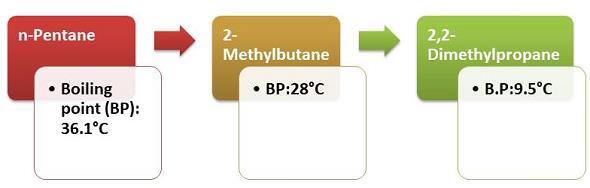
Figure 4: Exceptions to Boiling Point of Alkanes
The alkanes have a straight chain which results in the higher value of boiling point. This is, however, in comparison to the structural isomers noticed for alkanes.
The melting point is quite similar to the boiling point, noticed among the compounds of alkanes. Therefore, the melting point increases with an increase in the molecular weight of alkanes.
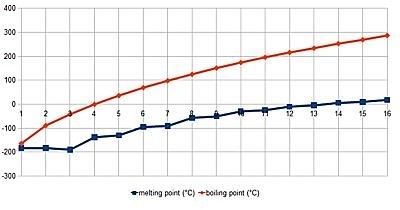
Figure 5: Melting Point
1840460mahesh, Alkanes boiling and melting point graph, CC BY-SA 4.0
The higher alkane is noticed to be solids and therefore it is quite difficult to break down intermolecular forces.
Other properties of alkanes include chemical properties. The chemical properties involve two major characteristic features of alkanes are combustion and halogenation.
The process of combustion is referred to as a chemical reaction that occurs between oxygen and substance. However, this reaction takes place when heat and light are given to it. The group of alkanes are high in combustion and instantly conducts the process in the presence of sufficient oxygen. The reaction is expressed as,
CH4+2O2→CO2+2H2O+energy
However, an exothermic reaction is noticed in the process of combustion of the alkanes. Therefore, alkanes are used in the production of fuels.
The reaction of Halogenation results in the formation of derivatives of hydrocarbons. This results in the substitution of one or more atoms of halogen from the atoms of hydrogen. The reaction of “Halogenation reaction is expressed as −
CH3−CH3+Br2→CH3−CH2−Br+HBr
In the aspect of industries, alkanes are considered to have high commercial usage. The application of alkane includes, as a major component of LPG or liquefied petroleum gas, and making of petroleum jelly. More to this, the alkanes are used in jet fuels and due to the high viscosity the alkanes are used as lubrication oils, in making candles and making of synthetic polymers.
In this tutorial, the focus has been given to comprehending the properties of alkane compounds. This not only includes physical properties as well as chemical properties. The structure and the alkanes are discussed that show the tetrahedral structure and it has sigma bonds among its carbon and hydrogen atoms.
Q1. What is the properties noticed for alkanes?
Ans. Alkanes are known to be the simplest in the family of hydrocarbons. These compounds have carbon and hydrogen that consist of bonds that include carbon-hydrogen and carbon-carbon. These compounds are seen to be quite less in their reactivity level together with very less biological activities. More to this, alkanes are unique as they have no colour in them and they are as well odourless.
Q2. What is considered as the physical state for higher alkanes?
Ans. Higher alkanes are most times used as literal sense at times that simply means alkanes have a higher number of atoms of carbons. Another definition states that higher alkanes are considered as n-alkanes. These n-alkanes are seen to be solids under natural conditions.
Q3. What are the varied types of alkanes found?
Ans. In the field of chemistry, majorly three kinds of alkanes are found that involve, the straight chain of alkanes, the branched chain of alkanes and lastly, cycloalkanes.