

Uses of amines refers to its applicability in textile industries, medicinal industries etc. Ammonia is the main source of amines and one of the known organic compounds. Various products are derived from ammonia and amine is one of them. Amine, as an organic compound acts as starting agent in the syntheses of other organic and inorganic products.
Amine is a type of chemical compound sourced from NH3 or ammonia. It belongs to the organic nitrogen compounds in its functional group containing a lone pair of nitrogen atoms. Alkaloids are consists of various degree of amines in its structure occurs naturally. These compounds are found in some plants including histamine, epinephrine, norepinephrine, and dopamine.
The number of carbon bonds with nitrogen atoms classifies amines into different degree of molecules such as, tertiary, secondary, and primary. The compound acts as an ammonia derivative fulfils various purposes related to industrial sectors. Amine is used as a core product and also as by-product in pharmaceutical and agrochemicals industries.
Amines are classified into three types based on its degree of replacement of hydrogen atom from amine structure. These are: primary amines, secondary amines, and tertiary amines.
Primary amines are produced by the replacement of one hydrogen atom from the organic compound of ammonia. An aromatic group or alkyl does this task. Some instances of primary alkyl amines include methyl amine and amino acids.

Figure 1: Classification of Amines
If Aryl, alkyl or both organic substitutes two hydrogen atoms of organic compound of ammonia results in secondary amines.
When three organic substitutes are attached with ammonia based organic compounds then it is designated as tertiary amines.
Amines are organic compounds and related to the functional organic nitrogen group. It consists of a lone pair of nitrogen atoms. Hydrogen bonds are formed between amine groups and water molecules and results in water solubility along with an increasing its boiling points. The compounds which belong to a carbonyl group attached with amine group are designated as amino acid compound. Amino acid structure is expressed as R-CO-NR’R, here, amine group is in tertiary degree by replacing both hydrogen atoms with alkyl or aryl groups.
The main structure of this compound is responsible for its wide usage. Aromatic amine structures are formed by the attachment of amine groups to the benzene ring in single or multiple number results in alkaline characteristic of the benzene ring towards reduction reaction. When it comes to electron donation, the aromatic amine compounds as compared to aliphatic amines are less reactive due to delocalization of lone pair of Nitrogen atom towards benzene ring. Amine structure is composed of trivalent nitrogen atoms and an undistributed electron pair.
Primary amines are formed with one hydrogen atom replacement out of three by an aromatic element or alkyl group. The replaced hydrogen atom count is two then appears secondary amines. An organic substitute replaces all three hydrogen atoms produces tertiary amines. For saturated or unsaturated ring like structure of amines only can be classified into tertiary and secondary degrees.
Properties of amines and its structure contribute to its usage in various field. Its physical properties are as follows −
The boiling point of the compounds is high and soluble in water as it consists of hydrogen atoms in amine groups. The solubility decreases in water with increasing degree of the amine compounds that results in an increase of carbon atoms in the structure.
Variations of the number of carbon atoms in amine compounds differs its state of existence. Those compounds are in a typical gaseous state with less number of carbon atoms. It appears with a fishy odour. The amines are in liquid state with the content of three carbon atoms in the amine structures. The amines appear as solid substances when the carbon atom count is more than three.
The amines mostly have no colour. Atmospheric oxidation can help amines to obtain some colours.
Some important chemical characteristics of amines are described below −
Amines are basic. An increase of alkyl groups increases basicity of the amines.
Amines participate in various chemical reactions. The processes include acylation, alkylation, carbylamine reactions, and electrophilic substitution.
Amines, as dedicated sources of ammonia perform reactions with aryl sulfonyl chloride, nitrous acid and produces an oily substance in yellowish colour.
Amines are used for various daily needs and these have pharmaceutical uses.
Material dyes are prepared using amines as core material.
Amiens is used for treating other gases. Combustion of gases makes free of CO2 by using amines.
In garments and textile industries, azo dyes are critically required to treat substances like, nylon and leather. Amines are used to prepare azo dyes.
Lubricating oils and boilers in chemical processing industries can be prevented from corrosion by using amines.
Amines are used to increase solubility of herbicides. These are also known as emulsifiers.
It is also used for photograph development.
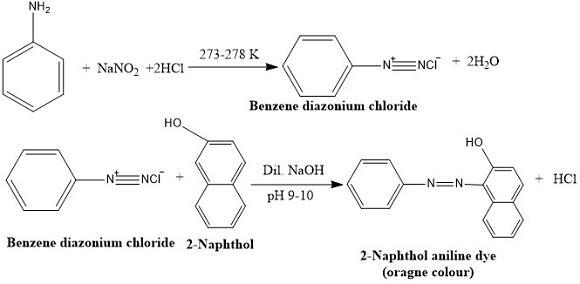
Figure 2: Amines are used for dye preparation in commercial scale
Demerol and morphine are two known painkillers contains amine as functional group with significant medicinal applicabilities. Amines have frequent usage in these medicines.
Novocaine is an anaesthetic drug. It has a high dependency on amines.
Benadryl syrups have the use of antihistamine diphenhydramine. One of its solvents is Amines.
Neurotransmitter in the body requires stimulants like serotonin. Amines are also a good stimulant for the same purpose.
Amino acids in the body can regulate the levels of vitamins and they can be sourced by amines.

Figure 3: N-Sulfinyl Imines in Preparation of Chiral Amine Derivatives are used in medicinal drug production
Professorattemple, Sulfinimine Applications, CC BY-SA 3.0
Alkyl or aromatic groups replace hydrogen atoms from organic compound of ammonia and these organic compounds are classified as primary, secondary, and tertiary amines. Amines are generally colourless and have a high boiling point. These compounds are used in chemical industries and pharmaceutical sectors.
Q1. What is meant by charge of amine?
Ans. Three substituents, one nitrogen atom, at least two hydrogen atoms, and a lone electron pair collectively considered as an amine functional group. The lone pair acts as electron donating agent towards any electron deficient group or solution. Hence, amines are electron rich and considered as negatively charged due to presence of electron pair. The binding of nitrogen occurs with 4 substituent and the nitrogen atom remains with a positive charge.
Q2. What is the functional group of amine?
Ans. Amines are derived from the processing of ammonia. Hence, it is called as organic compound of ammonia. An aryl p or alkyl group substitutes hydrogen atoms to produce amine. Therefore, primary amine consists of functional group of –NH2, secondary amines consists of functional group of –NHR, and tertiary amines exist with functional group of –NR3.
Q3. What are the factors that affect the basicity of amines?
Ans. Presence of electron-donating group in the same organic compound along with amine group increases basicity of amines, whereas, in presence of electron withdrawing group the organic compound with amine group reduces its basicity.