

The cohesive forces are the reason which does not allow the glass pane to stick. Surfactants in the detergents are reliable to reduce water’s surface tension. Water is used for cleaning different items regularly with a mixture of detergents as only water cannot clean something as desired. Surfactants are acting in the water as a spreading agent on the grease by connecting water to it, and when the water is disposed of, it brings all the dirt and other unwanted items with it. These behavioural changes are one of the consequences of the surface tension.
An attractive force which can apply to the available molecules of a liquid by the underneath molecules of the liquid is the surface tension. The inclines available on the liquid’s surface can influence the liquid to envision the shape to consist of the most inferior province of the surface (Kibron, 2022). Moreover, the liquid molecules can interact with the other nearby molecules. The molecules get attracted to the substance molecules which are also known as the cohesion force that can attract the molecules strongly with the adhesion force. The round shape of the water drops is the reason due to the cohesive force.
When an object drops on the surface of an element, it makes the application of force to make molecules of water apart from it. If the consequence of the object’s weight is incomplete to correspond to the attractive strengths between molecules in the consistency layer, the object will not penetrate the surface.
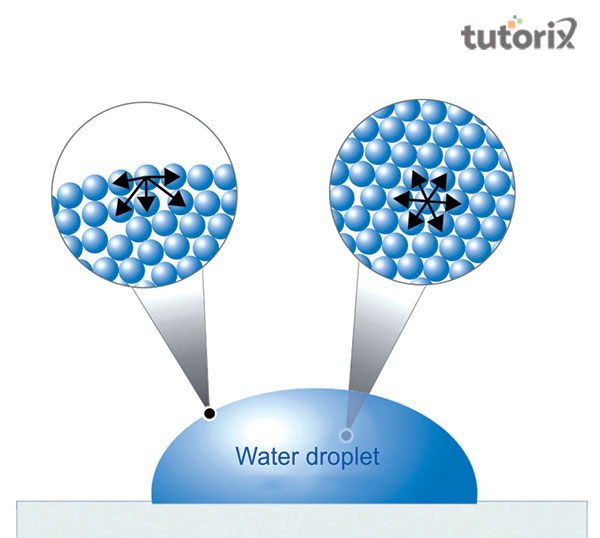
Figure 1: Surface tension of Water
The atoms of sodium (NA) or potassium (K) at the end of the hydrocarbon chains make the salts developed from these atoms. The molecules of detergents often have unique properties that can make the detergents mix with the surface of the water. This consequently decreased the tension force of the mixed water molecules with detergents.
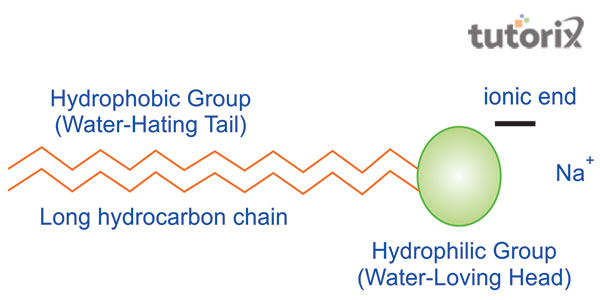
Figure 2: Detergent molecules for a surface tension
Detergents’ molecules are the hydrocarbon long-chain with an ionic cluster at one end. These molecules are usually bearing a negative charge and constructing it as an anion. Due to the present molecules in the detergents or soaps this charge is arrested by the contrasting charge of a action which is soluble, for instance, ‘Na+ or K’. However, long hydrocarbon chains are not interacting properly with molecules of the water, and numerous of them are actually squeezed out to the interfaces between the water and the air (Nagwa, 2022). The consequence of these molecules on the surface water is to considerably dilute the powers between molecules of water there, consequently, lowering the surface tension.
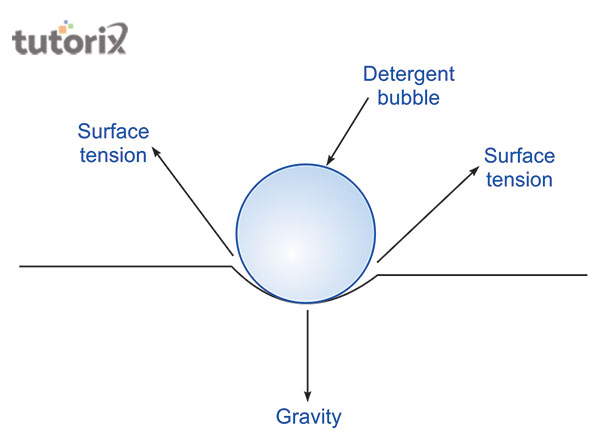
Figure 3: Surface tension while adding detergent
The result of detergent on the surface tension obtains a non-adhesive force to the molecules of the water. Due to this incident, the molecules do not stick to the glass’s surface. It is the result of the cohesive force on the molecules of water. The surfactants are the surface-active agent that makes changes in the molecules of water. Due to the detergents’ surfactants acting in the molecules of water, it becomes easy to determine the lower surface tension in the water.
When an amount of detergent dissolves in the water, the value of the surface tension of water starts to reduce. This functional component of changing the value is followed by the method capillary rise (Qazi et al. 2020). Due to this incident, detergent can spread in a larger provenance of materials that are dipped in the mixture to clean thus, it is known as the cleaning agent.
The following factors are affecting the surface tension of water by adding the detergents of soap and the application of the cohesive force can be found easily.
It is an exceptional aspect that assists to decrease the surface tension of the following liquid. The surfactants connect with a water molecule and reduce its earliest surface tension.
It transforms the elements of surface tension. When any random chemical is combined with a material, it transforms the surface tension. A surfactant in the molecules of water is the best instance of chemical expansion like adding detergent to minimise the value of the water’s surface tension.
It has a direct influence on the surface tension value. Oxidation is one of the natural ingredients that can appear due to the available amount of oxygen in the environment and it assists to reduce the earlier value of the molecule's surface tension (Song et al. 2021).
The temperature is an effective factor that can initiate the reduction of the surface tension in water. The molecules inside the mixture of the water and detergent can become more functional and make the increment of the temperature (Lyubimova et al. 2021).
In this tutorial, a discussion has been expressed on the subject of how the surface tension of water gets reduced after adding a small amount of detergent to it. The chemical present in the detergent or liquid soap can transform the molecules of water. Specifically, the bubbles that are formed due to the reaction of the Surfactant are reliable to reduce the surface tension and apply the gravitational forces. The attractions among the liquid molecules can be blamed on the transformation of the surface tension values of water.
Q1. What are surfactants?
Ans. Surfactants are one of the types of chemical compounds that are used to reduce the value of the surface tension of the applied item which can be liquid or gas.
Q2. What is the ideal fluid?
Ans. A fluid which cannot be compressed and the amount of the velocity does not fall after adding any different substance is usually referred to as an ideal fluid. Moreover, an imaginary fluid does not exist in the real world.