

Surface tension can be defined as the tendency of liquid surfaces to shrink into possible minimum surfaces in real time. In general, the small liquids in this process can shrink into the smallest feasible surface area. Here, the liquid can be in different states on the molecular level starting from the surface area to the centre of the liquid. Surface tension is determined by the attractive forces of solids, liquids and gases in their closest proximities.
Surface tension is the required energy needed for increasing the surface area of the liquids in real time. It can also be defined as the property of other liquids for resisting the force of the liquid surfaces. Commonly, surface tension acts as the barrier between the liquids and foreign things. This has earned it the property of a force that helps in the binding of liquid molecules to one another.

For example, if a drop of liquid is dropped on the floor, it immediately touches the floor due to surface tension. In addition, the soaps and detergents are another example of surface tension in daily human lives (Pan et al. 2018). When a splash of water is applied to the detergents, they start to make bubbles and expand in that form. This is a prime example of surface tension in real-time.
A molecule is tugged equally in almost all the directions by the liquid molecules caused by the cohesive forces. The surface tension of a liquid molecule generally increases when the liquid and the molecule meets due to cohesive interactions. So, it can be said that cohesive interaction is a prime reason behind triggering surface tension for liquid molecules. In a bulk of liquid, the molecules are commonly surrounded by the neighbouring molecules which eventually pull each other equally from all the directions with a net force of 0. As the molecules present in this form are not surrounded by the identical molecules, they are characteristically pushed in the inward direction.
Apart from these causes, surface tension is further affected by other factors like temperature, ‘impurities and surfactants. Characteristically, when the temperature tends to increase, the surface tension of liquid molecules starts to decrease. Similarly, if the soluble liquid is high in impurities, it starts to increase the surface tension successfully. Further, if water is mixed with surfactants like hand soap, it starts to lower the surface tension of the water and breaks the liquid particles into pieces.
In water, two types of molecules can be found commonly, the exterior molecules and the interior molecules. The interior molecules are generally attracted to the neighbouring molecules that surround them from every possible side. The exterior molecules, on the other hand, are attracted to the surface molecules that reside in the below surfaces (Polyakov & Schweitzer, 2018). This enables the energy state of the molecules to lower the tension on the interior side of the molecules compared to the exterior side of the molecule.
Images Coming soon
Initially, the molecules attempt to maintain minimum surface areas then, it starts to allow more molecules to possess a lower energy state. This triggers the surface tension within the liquid molecules in real-time. The water molecules generally start attracting each other due to the polar property of water. The molecules in the surface area of the water experience a net attraction towards the other molecules present in the bulk form of liquid. Here, the interior experience experiences uniform attractive focus that eventually creates surface tension.
The cohesive and adhesive forces are directly related to the surface tension of liquid molecules. The cohesive forces hold the body of the liquid with the adhesive forces and the minimum surfaces, especially when the liquid spread out. When the cohesive forces appear stronger than the adhesive forces, the water body then starts to maintain its shape. On the contrary, an opposite phenomenon happens when the liquid spread out and maximizes the surface area.
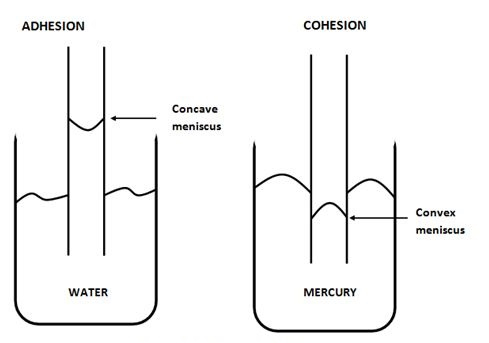
Any substances that can be added to a liquid that allows it to increase the surface area. This increase in the surface area is called the wetting agent. For example, when adhesion happens in water, the concave meniscus appears higher than the level of water. Mercury, on the other hand, causes cohesion where the convex meniscus stands lower than the level of mercury within a beaker.
Surface tension acts as the required energy that increases the surface area of the liquid molecules. The phenomenon of surface tension eventually causes internal pressure, causing the compression of the liquid surfaces to the smallest area. The process is affected by various factors like temperature, surfactants and impurities that cause surface tension in the lower levels of liquid molecules in real time. In addition, the cohesive and adhesive forces influence the occurrences of surface tension for liquids.
Q.1. Which properties are capable of affecting surface tension?
Ans. The intermolecular forces are capable of affecting the surface tension in real-time. It depends on the nature of the liquid to some extent along with the temperature and the surrounding environment.
Q.2. What causes the breaking of the surface tension?
Ans. The polar property of water is affected by the molecules of water by one another. In this scenario, the certain amount of energy within the surface can cause the breaking of the intermolecular bonds of materials, eventually influencing surface tension in the process.
Q.3. Can surface tension cause an increase in viscosity?
Ans. The increase of viscosity is interrelated with the surface tension of material in real-time. When the intermolecular bonds of water remain unchanged, the viscosity of the material starts to increase, eventually affecting the increase of viscosity caused by surface tension.