

The thermal conductivity of any given material is associated with the ability to conduct heat energy. There are certain elements that display a very low range of thermal conductivity in the heat energy that is being transferred, in such cases the rate of transference of heat occurs at a very slow pace. Metals in general display high conductibility in terms of electricity and heat alike. Therefore metals are known to display a very high range of thermal conductivity.
Metals, in general, have very high conductibility which is accentuated by the aspect of possessing an extensively high amount of free electrons. Therefore the heat conduction that exists in the metals leads to the aspect of the electronic conduction. In the cases of metals, the free electrons that exist within them are able to move freely in the solid body and thus are able to transfer the thermal energy at a substantially high rate in comparison to the insulators.
It is also needed to be considered that among the simplest of the metals, the electrical conductors also display prime thermal conductivity. The aspect of thermal conductivity is analogous to the property of electrical conductivity. The lattice and electronic conduction are the key factors that drive the rate of heat conduction that occurs in the metals. The relation between electrical conductivity and thermal conductivity is postulated by Wiedemann Franz law.
The aspect of thermal conduction is subdivided into three distinct categories of distinct features, that is, the molecular vibrations that take place in the gaseous and liquid forms, the lattice vibration of the solids and the electrons that exists in the metals. Multitudes of thermal conduction processes that exist in the metals are regulated by the aspects of the collisions of molecules, electrons for metals in the gaseous state and solid-state are also responsible for the process of conduction associated with the lattice vibrations. In short, the free electrons are essentially responsible for making metal an excellent conductor.

Figure 1: Thermal conductivity of metals
The most important factor that contributes to the high conductivity in metals is the free-flowing electrons that exist within the material.
The atoms present in the metal give out valence electrons that chemically react with various non-metals and tend to form oxides and salts. The metal ions thus form cations existing in the solution. This special bonding helps in making the metal and metallic alloys effective conductors of heat.
The metal solids tend to have various bonded electrons that share their valence electrons. As opined by Köbler (2017), this aspect of sharing electrons creates a lot of various moving conduction electrons that help in carrying both the heat and electrical charge. Therefore, unlike the electrons that exist in the covalent bonds, the valence electrons existing within the metal are able to move without hindrance through the lattices of the metal, these valence electrons carry heat without getting locked to an atomic core.
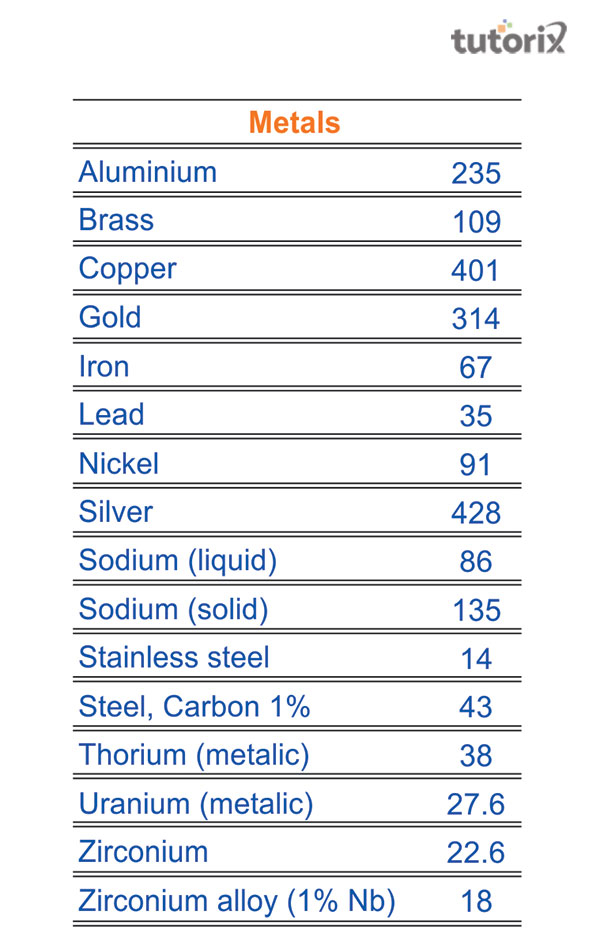
Figure 2: thermal conductivity of metals
Metals and the alloys, which are formed by the mixture of various alloys are responsible have a high thermal conductivity which allows them to be used in various industries like engineering, electronics, household products and construction of medical and laboratory devices. Copper is generally used in various equipments for its high electrical and thermal conductivity’ (nuclear-power 2022). It is also cost-efficient and thus is extensively used in electrical wiring all around the world. Lead is also a metal which is useful for making batteries and cable sheathing.
According to Xu et al. (2018), the thermal conductivity in metals is important that is useful for designing various structures. Metals are their property of thermal conductivity and are responsible for designing various products that are useful in designing the innovations of various industries.
This aspect is thoroughly crucial for the security and efficiency of these products and multiple constructions. Silver possesses the highest amount of thermal conductivity and thus is used in various delicate pieces of equipment. The aspect of conductivity of metals is utilized for effective electrical wiring, used in medical and laboratory devices. In order to know the type of matter involved, the aspect of electrical and thermal conductivity is used.
The aspect of thermal conductivity is one of the crucial properties of the metal which is accentuated by various other aspects. The most important feature, however, relies on the fact that the metals contain numerous amounts of free electrons that help in the efficiency of the thermal conductivity of the metals. The rate of transference of heat existing in the metals is utilized by different industries for the construction of different devices and their associated safety.
Q1. How does temperature affect the thermal conductivity of metals?
The aspect of thermal conductivity necessarily depends on the electronic effect of the pure metals and the alloys. The rise in temperature marks both the number of free electrons and an increase in lattice vibrations. Therefore the thermal conductivity of metals is supposed to increase.
Q2. What is Wiedemann Franz law?
The Wiedemann Franz law states that the ratio of thermal conductivity and electrical conductivity is proportional to the temperature of a particular specimen. It concludes that the increase in temperature increases the thermal conductivity of the material while the electrical conductivity decreases.
Q3. What are the limitations of Weiderman Franz law?
The proportionality as established by the law is not true in all the cases of temperature ranges; it is only applicable in cases of very steep temperatures. Materials like beryllium and silver do adhere to this law.
Q4. Which factors affect the thermal conductivity of metals?
The thermal conductivity in metals is accentuated by the presence of numerous amounts of free electrons. The heat transfer in the solids is composed of two particles, lattice and electronic conduction of the material.